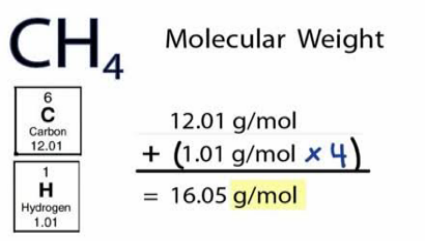
Molecular weight is also known as molecular mass is the total mass of substance molecules based on 12 due to the fact the atomic weight fit carbon is-12. It has to do with the adding of atomic weights of different atoms in order to make up one molecular formula for any substance. For example, the molecular weight of a hydrogen molecule with a formula H2 is 2 ounces rounded off. While organic molecules that are more complex could be in millions and these include polymers, molecules, proteins, etc.
Molecular weight is the measure of the total of all atomic weight values of an atom in any molecule. This structure is used in chemistry in order to determine the stoichiometry in any chemical equations or reactions. It has two abbreviations which can be MW or M.W. It can possess no unit or express in Daltons (Da) or atomic mass units (amu).
Atomic and molecular weight is mostly expressed as a relative to mass if any isotope carbon-12 and is given a value of 12 amu. A good reason why the atomic weight of carbon isn’t exactly 12 is due to the mixture of carbon isotopes.
Molecular Weight versus Molecular Mass
Although often used interchangeably, could there be a difference between molecular mass and molecular weight in the world of chemistry? These terms mean different things or can be used for the same thing all depending on the position they are in or who is making a reference on either.
Molecular mass is explained as the measure of mass while molecular weight is defined as the force which acts on this molecular mass. A better term for both these terms has a name in the world of chemistry and that is “Relative Molecular Mass”.
Calculating Weight of Molecular Isotopes
When calculating specific isotopes of an atom, it is necessary to use the atomic weight of that particular isotope than having to use the weighted average which can be gotten from the periodic table. A good example is the use of hydrogen and deuterium. If you are making use of the latter instead of the former, you are to use 2:00 instead of 1.01 for your atomic mass of that particular element. Generally, difference berries atomic weight of an element and also the once specified isotopes are relatively minor but then they are very essential in certain calculations you do not want to afford to make mistakes in.
Molecular Weight Determination
The empirical data present on the molecular weight of any compound depends on the size of that particular molecule. A common way to find the molecular mass of small to medium molecules is through the use of mass spectrometry. However larger molecules, as well as macromolecules such as Proteins and DNA, are found with a viscosity of light scattering methods.
Molecular Weight Calculator
Using a Molecular weight calculator is a good way to calculate and determine your molecular mass in a simpler and easier method. It saves you more time and gives you a lot more accurate answers.